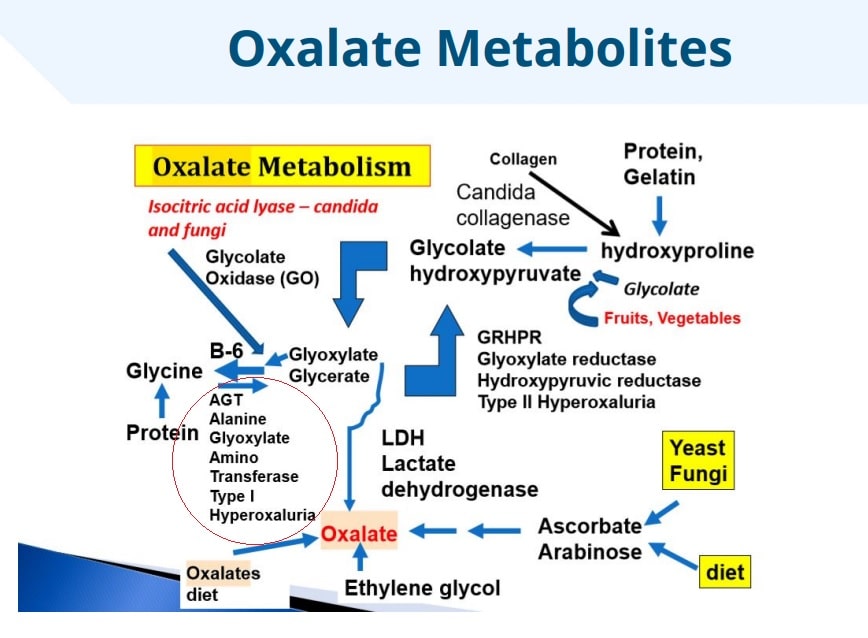
About thirty to forty percent of oxalate comes from the diet and the rest comes from what your body makes itself. This is of course, my opinion and of many other functional doctors I have consulted with over the years.
Oxalic acid in the diet is first converted to glycolate, then glyoxylate, and then at this point glyoxylate can either bind to a mineral to form oxalate or it can be transferred and form glycine.
Things get complicated when you have type 1 hyperoxaluria.
AGXT defects
If you have a genetic deficiency in the enzyme AGXT, the glyoxylate primarily forms oxalate because reduced amounts of AGXT do not function adequately to override this process. This is type 1 hyperoxaluria.
One in five people in the population has this genetic variant in which they cannot detoxify this compound. Instead, it predominantly forms oxalate in type 1 hyperoxaluria.
It has been found that one third of the people with oxalate toxicity have this genetic variant, and 53 percent of them are likely to have acute, very severe neurotoxicity versus only 4 percent in those with normal genotype expression. Probably a high percentage of people who have kidney stones are in this group of 20 percent of individuals with this genetic variant of type 1 hyperoxaluria.

Clues in Blood Sugar Chemistry
One of the body’s energy production factories called glycolysis is inhibited by oxalates. I often see this when measuring blood chemistry, presented by lower levels of lactate dehydrogenase enzyme and other clues in blood sugar chemistry. LDH is an enzyme found in nearly all cell types where it regulates the homeostasis of lactate and pyruvate as well as glyoxylate and oxalate metabolism.
LDH is the enzyme involved in the catalytic conversion of pyruvate to lactate using the cofactor NAD+ for exercising muscles in the presence of NAD/NADH that occurs in every cell. It is released in the bloodstream and the liver takes it up and converts it back to glucose, which is released into the bloodstream to be taken up by recovering muscles or red blood cells.
This enzyme is also involved in converting glyoxylate to oxalate in the cytoplasm, the last stage of oxalate metabolism in the liver.
LDH is involved in carb metabolism, cellular transport of chloride with glucose, and glucose with zinc and Na. This enzyme is often lowered when there is low serum glucose or a downregulation in glycolysis. It is often elevated when there is tissue destruction. Interestingly, inhibition of LDH has been used as a treatment for hyperoxaluria.
The enzyme pyruvate kinase is involved in the last step in the body’s energy production and is strongly inhibited by oxalate.
I have seen patients with both low and elevated LDH levels and high oxalates as well. This is why it is critical to run many assessment, not just blood chemistry, to assess what may be affecting your own biochemical patterns.
How thiamine is involved
Even a marginal thiamine deficiency can affect your B6 levels. This is critical, because B6 is very important for AGXT. Investigating this is not an easy feat and it doesn’t often show up in lab testing. It is important to investigate and address this if you have type 1 hyperoxaluria.
Some clues include:
- Diminished activity of thiamine dependent enzymes- such as transketoloase, pyruvate dehydrogenase, branched chain ketoacid dehydrogenase, and alphaketoglutarate (some of these are on the Nutreval and Genova OAT test)
- Low transketolase activity which drive down NADPH– which can then lead to reduced glutathione status. NADPH is used as a cofactor by GSR (glutathione disulfide reductase) to reduce oxidized glutathione. Riboflavin is also involved, as it is a cofactor GSR. Oxidized glutathione is pro-inflammatory. Glutathione also requires vitamin C to regenerate glutathione.
- Note-Low glutathione can lead to poor detoxification of glyoxal and methylglyoxal and this can lead to increased carcinogenic protein adducts. This is why both Vitamin C in the form of Camu Camu and Niacinamide are added to Vykon and I ask my patients to take that for 30-60 days before starting glutathione supplements.
- Diminished pyridoxal kinase activity (PK). PK is the enzyme that converts the inactive form of B6 into its active form P5P. Some people may have a polymorphism in this enzyme too. Low P5P prevents glyoxylate from being converted back to glycine, leading to high oxalates. Many mistakenly believe that ONLY low B6 is responsible for high oxalates- but there is evidence that thiamine deficiency is also a big contributor.
Remember, AGXT requires sufficient B6 to convert glyoxylate to glycine, instead of oxalate.
The critical factor here is that AGXT works much better in the presence of high amounts of vitamin B6. This is another one of the holistic treatments for people with kidney stones. This also applies to type 1 hyperoxaluria. In fact, vitamin B6 treatment is also used by the mainstream medical community for people with kidney stones.
Copper “toxicity”
You may have heard excessive vitamin C can worsen an oxalate problem. The real problem with vitamin C is the metals that the person may be taking. If one is taking high amounts of copper or iron, these can accelerate the breakdown of vitamin C to form oxalates. Someone with high copper or iron levels may be at risk for higher oxalate formation if also supplementing with vitamin C. The vitamin C may be broken down to form dehydroascorbate (DHA) and then oxalate. This is why I caution people with taking high doses of vitamin C over 250mg. Also, there may be some hidden copper toxicity on the tissue level, which we can identify with HTMA testing and patient history.
Know your Copper and iron status
Knowing your copper and iron status can be very important, especially if you suspect type 1 hyperoxaluria. If, for example, you have copper pipes in your home and you have acidic water, that water will dissolve your copper pipes so that most of the water coming out of your faucet will contain high amounts of copper. In turn, the high copper may cause you to degrade your vitamin C. There is a problem with forming extra oxalates, but this also means that even if you take high doses of vitamin C, it may not be useful because the copper can degrade it so rapidly. It is the free copper that is so toxic. Copper is bound to a protein in the blood called ceruloplasmin
To properly assess your status, you need to run a Hair Tissue Mineral Analysis, Plasma and RBC Copper, Plasma and RBC Zinc and Ceruloplasmin. Iron assessment includes Hemoglobin, RBC, iron, ferritin, Transferrin Saturation and TIBC.
What color is your blood.?
In lab studies, donors who had high copper had serum that was green. Scientists could pick out the samples with high copper just by looking at them in the freezer and choosing the green color. Almost invariably the samples were from women who were on birth control pills. The estrogen causes the body to make more of this protein.
We test this copper-zinc profile in the Great Plains Laboratory and the most important value is the free copper. This copper-zinc imbalance is important in almost every chronic disease: ADD, schizophrenia, arthritis, chronic fatigue, and many others. When you have too much copper and not enough zinc, vitamin C will not be utilized, it will be destroyed.
*DO YOU HAVE PYLORUIA?
This can also cause zinc and B6 deficiency, and high levels of free “unbound copper. Some believe that this “unbound” copper can chelate with oxalates and then be deposited in your tissues.
Pyroluria is a condition that has been known for several decades. It is sometimes referred to as mauve factor, KPU, kryptopyroluria and Hydroxyhemopyrrolin-2-one, or HPL. This condition is potentially very serious, and may have numerous biochemical implications
Pyrroles are synthesized during hemoglobin production in the body. They can be thought of as a byproduct of hemoglobin, when hemoglobin is synthesized. While it is normal for some pyrroles to be produced as a hemoglobin byproduct, certain individuals tend to produce an excess.
2 critical nutrients!
Pyrroles have an affinity for binding to and causing a high depletion of 2 critical nutrients:
- Vitamin B-6
- Zinc
Most cases of pyroluria are familial, though high levels of pyrroles may appear in response to increased levels of oxidative stress. For example, elevated urine pyrroles are often seen in chronic lyme disease.
Pyroluria can be associated with varying types of neurological symptoms, as well as mental and behavioral health symptoms and conditions. Many clinicians report the resolution of symptoms with the simple treatment of B6 and zinc when elevated urine pyrroles present. It should be noted, however, that pyroluria may be a part of a larger clinical presentation.
Much of the current research on the effects of mauve factor (another name for pyroluria) on the biochemistry demonstrates the correlation between glutathione depletion and this condition. Additionally, this same research suggests a role of intestinal permeability in the development of mauve factor.
Curiously, antibiotic treatment has reported the ability to decrease urine pyrrole excretion, indicating a possible microbial role in the development of pyroluria.
The role of B6 and zinc
A depletion of zinc and B-6 has direct implications on the function of the nervous system and brain. It is for this reason that pyrroluria is strongly associated with neurological conditions, bi-polar, down syndrome, ADHD, alcoholism, anxiety, depression, schizophrenia, and involuntary muscle twitching and abnormal motor function.
Vitamin B-6
In the instance of an increasing production or pyroles, the pyroles have an affinity for binding to aldehydes such as B-6.
Vitamin B-6 is critical for several biological and metabolic functions:
- Neurotransmitter synthesis
- Gene expression
- Synthesis of hemoglobin
- Histamine production via histamine decarboxylase
- Metabolism of amino acids, lipids and glucose
- Methylation status- which can affect expression of genes
- DAO enzyme- vitamin B6 is a cofactor for DAO.
- Oxalate degradation via AGXT
The functions of the brain and nervous system are chemically dependent upon B-6 status. Vitamin B-6 plays a critical role in the synthesis of 5 essential neurotransmitters: norepinephrine, serotonin, dopamine, epinephrine, GABA.
B-6 deficiency may alter the expression of genes. This may be due to the essential role that B-6 plays in a biochemical pathway referred to as methylation, which greatly affects DNA and RNA activities.
Additionally, B-6 is critical for the synthesis of a class of lipids called sphingolipids. These lipids are critical for cell signal transduction, and neural health. Abnormal lipid values have been identified in individuals with elevated urine pyrroles. This may be especially true for an omega 6 lipid, arachadonic acid.
In addition to causing a depletion of Vitamin B-6, increased pyrrole production will also tend to deplete zinc.
Zinc is an essential trace element that activates several hundred enzymatic reactions. These reactions are fundamental to life and biological activity. Some of the activities that zinc are involved in:
- DNA & RNA synthesis
- Gene expression
- Nervous system function
- Immune function & immune signaling such as cell apoptosis
- Neuronal transmission
- Brain function
- Zinc possesses powerful anabolic activities in the cells
- Formation of zinc proteins known as “zinc fingers”
- Zinc is essential for blood clotting and platelet formation
- Zinc is involved in Vitamin A synthesis
- Folate is made available through zinc enzyme reactions
- Along with copper, Zinc makes up the antioxidant enzyme system, ZnCu superoxide dismutase
- Steroidal hormone synthesis
- Growth & development of children
- Testosterone and semen formation
In certain instances, if zinc is depleted, copper levels may elevate. This is due to the intrinsic relationship that exists between these two trace elements. The biochemical implications of copper toxicity are well established. Free, unbound copper ions may create a tremendous amount of havoc and toxicity for biological functions.
A test you must do to rule this out is the Kryptopyrrole test https://www.greatplainslaboratory.com/kryptopyrrole-test
A lot of the symptoms of oxalate dumping (e.g. BMs that feel “on fire”, burning in your stomach, overall burning) are also sometimes symptoms of copper dumping. Oxalates could also have been binding up zinc and possibly iron, due to their negative charge.
Pyloria can cause low B6 activity and promote more endogenous oxalates.
AGXT ENZYME
This enzyme participates in alanine and aspartate metabolism and glycine, serine and threonine metabolism. Running a Nutreval panel can identify if the activity of these enzyme markers are high or low.
Prevention of primary manifestations of AGXT:
- Maintenance of high fluid intake;
- Pyridoxine supplements B6) for those who are pyridoxine responsive;
- use of potassium or sodium citrate, pyrophosphate-containing solutions, or thiazides to minimize stone formation.
Surveillance: Regular renal ultrasound and fundoscopic eye examinations; ongoing urinalysis; regular measurement of glomerular filtration rate (GFR) and creatinine levels.
In those with reduced GFR: regular measurement of plasma oxalate when possible.
Agents/circumstances to avoid:
- Dehydration
- foods high in oxalate (e.g., chocolate, rhubarb, and starfruit);
- megadoses of vitamins C and D
- loop diuretics.
What about histamine?
A new study gives some insights about a metabolic relationship between oxalate and histamine. It involves the enzyme ALT, which is a B6 dependent enzyme that is called a “liver enzyme” and is monitored as a marker of liver stress. It acts in the cytosol, whereas the enzyme AGXT, which is broken in Primary Hyperoxaluria Type 1, similarly acts on alanine, but it is located in the peroxisome.
ALT, this paper found out, is also important for routing histidine towards forming glutamate needed in the ALT reaction and this pull takes histidine in an opposite direction to forming histamine. The liver enzymes ALT and AST require vitamin B6 as a cofactor. Interestingly, the enzyme needed to reroute histidine to glutamate requires B3 as NAD is a cofactor for this reaction.
Here we see again the importance of B6 which is needed by AGXT and ALT because it will take alanine or serine into the direction that relieves the body from a buildup of glyoxylate and that is important to keep glyoxylate from turning into oxalate. B6 is our friend in helping our transaminases to work.
Dr. Clive Solomon was behind a lot of this oxalate research.
Clearly, the model Dr. Clive Solomons spoke about and many of us who have reduced oxalate have seen happen in our own bodies, is when oxalate seems to increase histamine release. We don’t really know which cell types this involves, but this paper gave a few hints of where to look for where this happens but the writers of this paper clearly did not have an oxalate-induced release of histamine in their model.
https://www.thevpfoundation.org/effective_treatment.html
Primary hyperoxaluria type I (PH1) is an autosomal-recessive inborn error of liver metabolism caused by alanine:glyoxylate aminotransferase (AGT) deficiency. In silico modeling of liver metabolism in PH1 recapitulated accumulation of known biomarkers as well as alteration of histidine and histamine levels, which we confirmed in vitro, in vivo, and in PH1 patients. AGT-deficient mice showed decreased vascular permeability, a readout of in vivo histamine activity. Histamine reduction is most likely caused by increased catabolism of the histamine precursor histidine, triggered by rerouting of alanine flux from AGT to the glutamic-pyruvate transaminase (GPT, also known as the alanine-transaminase ALT). Alanine administration reduces histamine levels in wild-type mice, while overexpression of GPT (ALT) in PH1 mice increases plasma histidine, normalizes histamine levels, restores vascular permeability, and decreases urinary oxalate levels. Our work demonstrates that genome-scale metabolic models are clinically relevant and can link genotype to phenotype in metabolic disorders.
Another breakthrough!
From a patient, indicating the importance of the higher doses of B6. However, this can be trick, as it can push up HMNT pathway as a cofactor and this can increase histamine. As always, I expect everyone to take it SLOW and SLOW with increasing B6.
“I was already taking 100 mg B6 and several doses of 25 mg P5P — the active form of B6 — throughout the day. My doctor wanted to increase my B6 to 300 mg but I stopped her bc I wanted the freedom to take little doses of P5P instead of having a high dose built into my compound supplement. However, I think she was right.
I have started increasing my B6 by drinking it in a liquid form (mixed with water) throughout the day. Right away I could tell it was really, really helping. B6 is a funny vitamin and for some reason some people need more P5P (the active form), and some people need more B6 — I have yet to find a good explanation of why this is. Some people — could be those with infections, or methylation problems, or who are deficient in mag or lysine — do not tolerate B6 well. I do not understand the mechanism behind those who react badly to it but I would be remiss if I did not mention that.
HOWEVER, there are also those “who react badly” to LOW B6, and in my opinion these low B6ers could be YOU and ME. Low B6 is KNOWN to play a role in endogenous production of oxalates, and in some cases — such as myself — I believe we are not supporting it enough.”
References
Lai, C., Pursell, N., Gierut, J., Saxena, U., Zhou, W., Dills, M., Diwanji, R., Dutta, C., Koser, M., Nazef, N., Storr, R., Kim, B., Martin-Higueras, C., Salido, E., Wang, W., Abrams, M., Dudek, H., & Brown, B. D. (2018). Specific Inhibition of Hepatic Lactate Dehydrogenase Reduces Oxalate Production in Mouse Models of Primary Hyperoxaluria. Molecular therapy : the journal of the American Society of Gene Therapy, 26(8), 1983–1995. https://doi.org/10.1016/j.ymthe.2018.05.016
Pagliarini, R., Castello, R., Napolitano, F., Borzone, R., Annunziata, P., Mandrile, G., De Marchi, M., Brunetti-Pierri, N., & di Bernardo, D. (2016). In Silico Modeling of Liver Metabolism in a Human Disease Reveals a Key Enzyme for Histidine and Histamine Homeostasis. Cell reports, 15(10), 2292–2300. https://doi.org/10.1016/j.celrep.2016.05.014