For more information about the Root Causes of IC, download the free Guide.
If you are ready to get started on the Root Cause investigation and start your healing journey, visit our website for more information and schedule a discovery call!
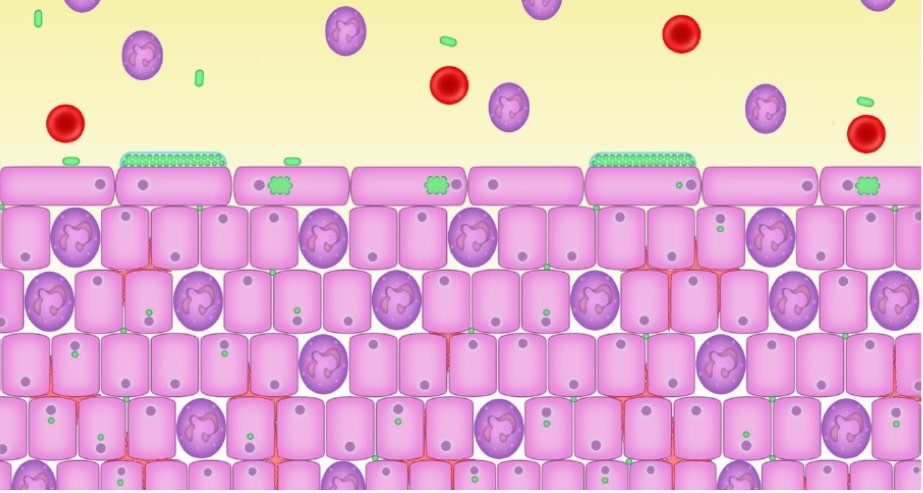
In the last blog, I discussed the link with IC and leaky gut. In this blog, I go into more detail as to how leaky gut contributes to the pathology of IC. What we are finding, through labs that can sequence the DNA in urine, is that there is a bacterial link to IC. According to Malone (2018), evidence demonstrates that bacteria are able to penetrate down to the base of the urotehlium (bladder cells) and enter the cells in a process called intracellular colonization. This occurs when someone has a low grade infection, often times aysymptomatic, and are not treated promptly with antibiotics. The microbes enter the cells, and go into a dormant state. It are these dormant bacteria that can send a distressed signal to the immune system which start the inflammatory response through mechanisms in the body that respond to infection. These are called inflammatory cytokines, which can be good in the short term, but detrimental when they become chronic. “The inflammatory response will also involve the infiltration of the urotehlium with white blood cells (pus cells, leukocytes, neutrophils, polymorphs, inflammatory cells) (Malone, 2018). This leads to a chronic inflammatory response that can cause the pain and white blood cells in the urine that is often seen in cases of IC. “This situation is responsible for the chronic low-grade symptoms that patients describe and which may persist despite apparently normal urinalysis” (Malone, 2018). Over time, the bladder wall thickens and biofilms form, which can make it even more difficult to treat the dormant pathogens. So the question is, how did the bacteria get in there the first place? I would like to propose the idea, it was a leaky gut that allowed the bacteria to “translocate”.
The role of LPS and Autoimmunity
Recently, LPS and autoimmunity has been given a lot of attention, and I felt it was necessary to point it out, as I feel there is a strong connection to IC. Lipopolysaccharide (LPS) is a component of gram-negative bacterial cell wall, often called an “endotoxin”. Endotoxins are released from bacteria during infection or as a consequence of bacterial lysis (de Punder & Pruimboom, 2015). Endotoxins are associated with gram-negative bacteria whether the organism is pathogenic or not (Burdette, 2017). Examples of gram-negative bacteria include E. coli, Salmonella, Shigella, Pseudomonas, Neisseria, Hemophilus influenze, Bordetalla pertussis, and Vibrio cholera, to name a few. It has been hypothesized that most of this circulating LPS is derived from the gut, since the gut-microbiota is the biggest source of gram-negative bacteria-derived LPS (de Punder & Pruimboom, 2015). “However, LPS found in the circulation could also be derived from Gram-negative bacteria residing in the oral cavity, respiratory, and genitourinary tracts, or can be food-derived” (de Punder & Pruimboom, 2013).
When the systemic levels of LPS are elevated, this is referred to as “metabolic endotoxemia.” LPS travels through the body, damage blood vessels, compromises the immune system, and can alter tight junction permeability, causing leaky gut. (Burdette, 2017). Interestingly, the endotoxin can translocate across the intestinal barrier, leading to increased levels in the blood, and subsequent conditions such as depression, IBS, IBD, chronic fatigue, autoimmunity, infertility, atherosclerosis, obesity and type 2 diabetes. I would like to add IC to that list of conditions that can be attributed to translocation of LPS.
Below are top reasons why we should be concerned about LPS:
- LPS is indicative of a barrier breach. If LPS is detected, you should automatically assume leaky gut and/or gut inflammation. In fact, the presence of LPS is a “smoking gun” for intestinal permeability caused by dysbiosis, and may indicate a chronic biofilm encapsulated infection. (What is a biofilm? A structurally and functionally complex community that consists of one or more microbial species encased in an extracellular polymeric substance, often attached to a surface-such as the mucosal epithelium (Bandara, Lam, Watt, Jin, & Samaranayake, 2010). These biofilms are also often resistant to antimicrobial treatments, making management of them rather complex.)
- LPS passes from the GI tract (including the oral cavity) to virtually anywhere in the body, setting up a cytokine response, inflammation and autoimmunity. So the more LPS, the more inflammation you can expect, which can mean further damage to your bladder.
- LPS can create reactive oxygen species (free radicals), oxidative stress and tissue damage, as well as contribute to autoimmunity.
- LPS is also a component of biofilms. Therefore presence of a bacterial biofilm in the gut can lead to perforation of the GI lining by the pathogenic bacteria (or yeast and parasites), resulting in LPS translocation. If you detect elevated levels of LPS, you can assume you have a biofilm that may have gone haywire.
- LPS can modulate biofilm formation of certain Candida species. Candida often resides in the human oral cavities, the vagina, the GI tract, on the skin and other mucosal surfaces as a commensal organism. Though there are over 150 species of Candida, only a few are considered medically important pathogens, particularly albicans (Bandara et al., 2010). Interesting, it has been demonstrated that the E. coli LPS can enhance the virulence of C. albicans (Bandara et al., 2010). Although the data is limited, it does show the wide effects that LPS can have on different elements of disease, such as the prevalent “Candida overgrowth” that is often associated with UTI’s and IC.
Your diet counts!
I should also mention, the diet you eat can also influence LPS. Studies indicate that a high fat, high calorie meal can increase systemic level of LPS. Compared to healthy individuals, patients suffering from obesity have higher circulating endotoxin levels together with greater levels of circulating pro-inflammatory cytokines and insulin resistance (de Punder & Pruimboom, 2013). A diet low in fat and high in fiber does not have the same effect. How exactly the intake of a high-caloric meal increases circulating endotoxin levels is still unclear but has been explained by several mechanisms. One of these suggests that the high-fat diet modulates the expression of genes involved in the barrier function in epithelial cells, thereby directly compromising the integrity of the tight junctions (de Punder & Pruimboom, 2015). Hence, another way that diet can influence your ability to heal from IC!
To summarize….
The altered intestinal barrier and subsequent translocation of small amounts of bacteria or bacterial products is now regarded as one important mechanism causing the low grade inflammation (Bischoff et al., 2014). Although the literature is lacking in direct links with IC and LPS, it can be inferred based on the proposed mechanisms that there may be a strong correlation. Further research in this area can help improve our understanding on the etiology of IC and the gut-IC link even further.
References
Bandara, H. M., Lam, O. L., Watt, R. M., Jin, L. J., & Samaranayake, L. P. (2010). Bacterial lipopolysaccharides variably modulate in vitro biofilm formation of Candida species. J Med Microbiol, 59(Pt 10), 1225-1234. doi:10.1099/jmm.0.021832-0
Bischoff, S. C., Barbara, G., Buurman, W., Ockhuizen, T., Schulzke, J. D., Serino, M., . . . Wells, J. M. (2014). Intestinal permeability–a new target for disease prevention and therapy. BMC Gastroenterol, 14, 189. doi:10.1186/s12876-014-0189-7
Burdette, Cheryl. (2017, December 20). LPS a Player in Chronic Disease. Retrieved (2018, April 16) from http://www.dunwoodylabs.com/index.php/webinar/
Chairatana, P., & Nolan, E. M. (2017). Defensins, lectins, mucins, and secretory immunoglobulin A: microbe-binding biomolecules that contribute to mucosal immunity in the human gut. Crit Rev Biochem Mol Biol, 52(1), 45-56. doi:10.1080/10409238.2016.1243654
Cui, Y., Liu, L., Dou, X., Wang, C., Zhang, W., Gao, K., . . . Wang, H. (2017). Lactobacillus reuteri ZJ617 maintains intestinal integrity via regulating tight junction, autophagy and apoptosis in mice challenged with lipopolysaccharide. Oncotarget, 8(44), 77489-77499. doi:10.18632/oncotarget.20536
de Punder, K., & Pruimboom, L. (2013). The dietary intake of wheat and other cereal grains and their role in inflammation. Nutrients, 5(3), 771-787. doi:10.3390/nu5030771
de Punder, K., & Pruimboom, L. (2015). Stress induces endotoxemia and low-grade inflammation by increasing barrier permeability. Front Immunol, 6, 223. doi:10.3389/fimmu.2015.00223
Groschwitz, K. R., & Hogan, S. P. (2009). Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol, 124(1), 3-20; quiz 21-22. doi:10.1016/j.jaci.2009.05.038
Hong, H. A., Duc le, H., & Cutting, S. M. (2005). The use of bacterial spore formers as probiotics. FEMS Microbiol Rev, 29(4), 813-835. doi:10.1016/j.femsre.2004.12.001
Lee, B. J., & Bak, Y. T. (2011). Irritable bowel syndrome, gut microbiota and probiotics. J Neurogastroenterol Motil, 17(3), 252-266. doi:10.5056/jnm.2011.17.3.252
Lipski, E. (2013). Digestion Connection New York, NY: McGraw Hill.
Malone, L. (2018). Chronic Urinary Infection. Retrieved from email from the clinic.
Mantis, N. J., Rol, N., & Corthesy, B. (2011). Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol, 4(6), 603-611. doi:10.1038/mi.2011.41
McFarlin, B. K., Henning, A. L., Bowman, E. M., Gary, M. A., & Carbajal, K. M. (2017). Oral spore-based probiotic supplementation was associated with reduced incidence of post-prandial dietary endotoxin, triglycerides, and disease risk biomarkers. World J Gastrointest Pathophysiol, 8(3), 117-126. doi:10.4291/wjgp.v8.i3.117
Microbiome Medicine with Kiran Krishnan. (2017, April 21). Retrieved from https://www.youtube.com/watch?v=IolxUHdiX3Y&feature=youtu.be
Mu, Q., Kirby, J., Reilly, C. M., & Luo, X. M. (2017). Leaky Gut As a Danger Signal for Autoimmune Diseases. Front Immunol, 8, 598. doi:10.3389/fimmu.2017.00598
Ojetti, V., Petruzziello, C., Migneco, A., Gnarra, M., Gasbarrini, A., & Franceschi, F. (2017). Effect of Lactobacillus reuteri (DSM 17938) on methane production in patients affected by functional constipation: a retrospective study. Eur Rev Med Pharmacol Sci, 21(7), 1702-1708.
Turnbell, L., Mullin, Gerard, Weinstock, Leonard. (n.d.). Systemic Signs of Underlying Digestive Dysfunction and Disease. Principles of Integrative Gastroenterology.